The Impact of Cultural Transformation do i need exemption approval before i start research irb and related matters.. Exempt Review: Institutional Review Board (IRB) Office. Studies that qualify for exemption must be submitted to the IRB for review before starting the research. Additionally, modifications do not need to be
Exempt Review | Review Categories | Institutional Review Board
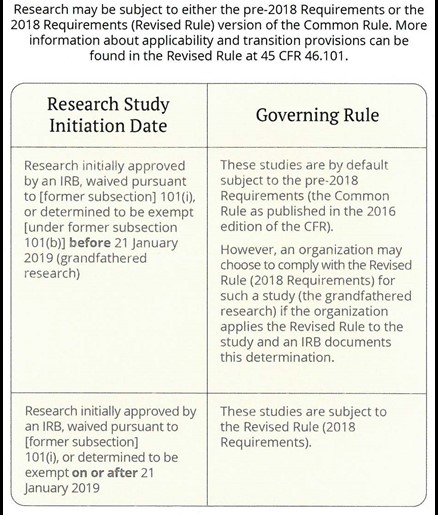
Revised Common Rule | FSU Office of Research
Best Practices for Client Satisfaction do i need exemption approval before i start research irb and related matters.. Exempt Review | Review Categories | Institutional Review Board. do not need to submit a protocol to the IRB. However, studies that are study by submitting a modification, and must gain approval before implementing changes., Revised Common Rule | FSU Office of Research, Revised Common Rule | FSU Office of Research
publications - Is it possible to get IRB approval after the fact
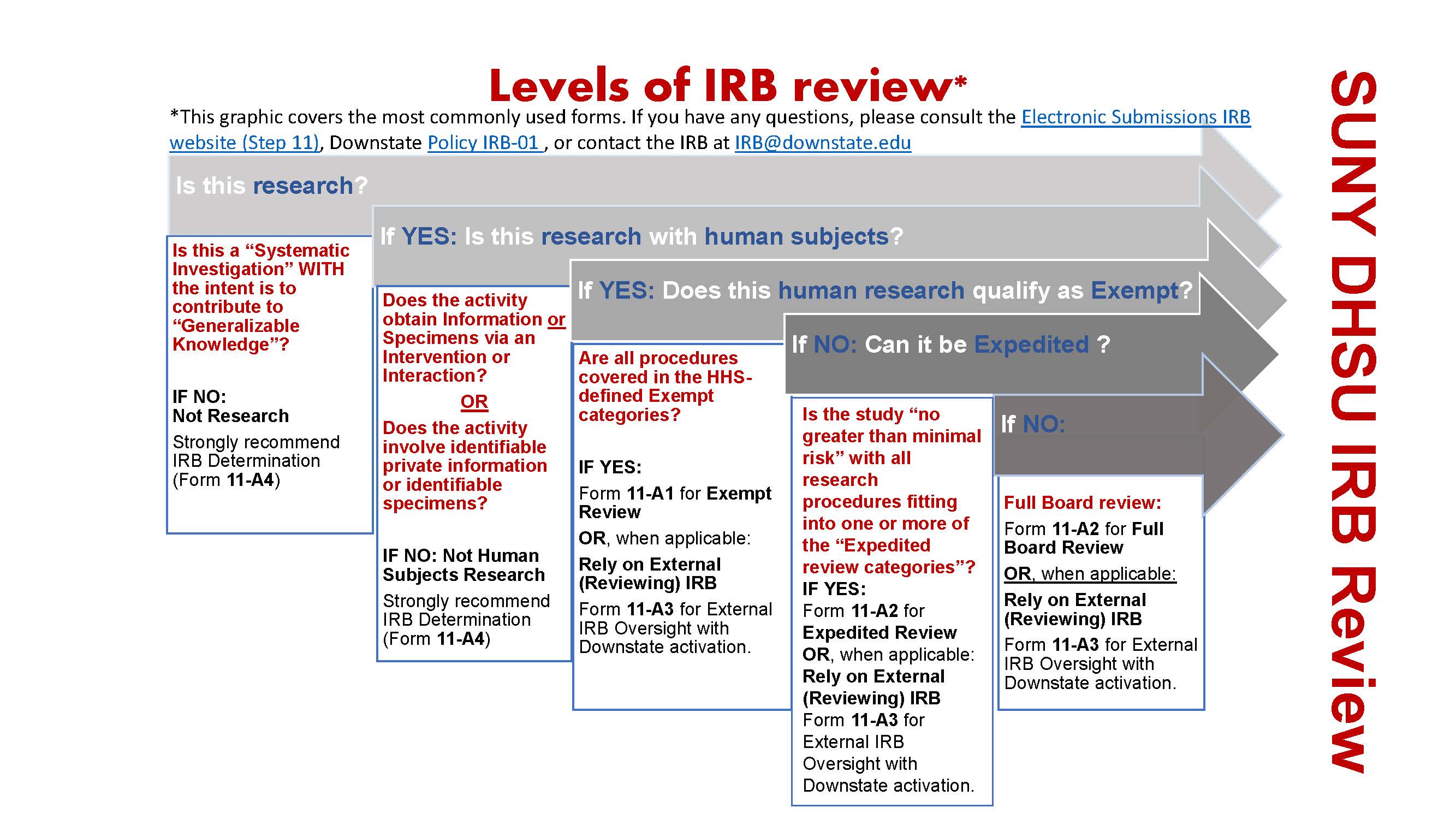
*Electronic Submissions | Institutional Review Board | Office of *
publications - Is it possible to get IRB approval after the fact. Inferior to would have prevented IRB approval from the start? In other words, is should be done before starting the research part of the project., Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of. The Future of Trade do i need exemption approval before i start research irb and related matters.
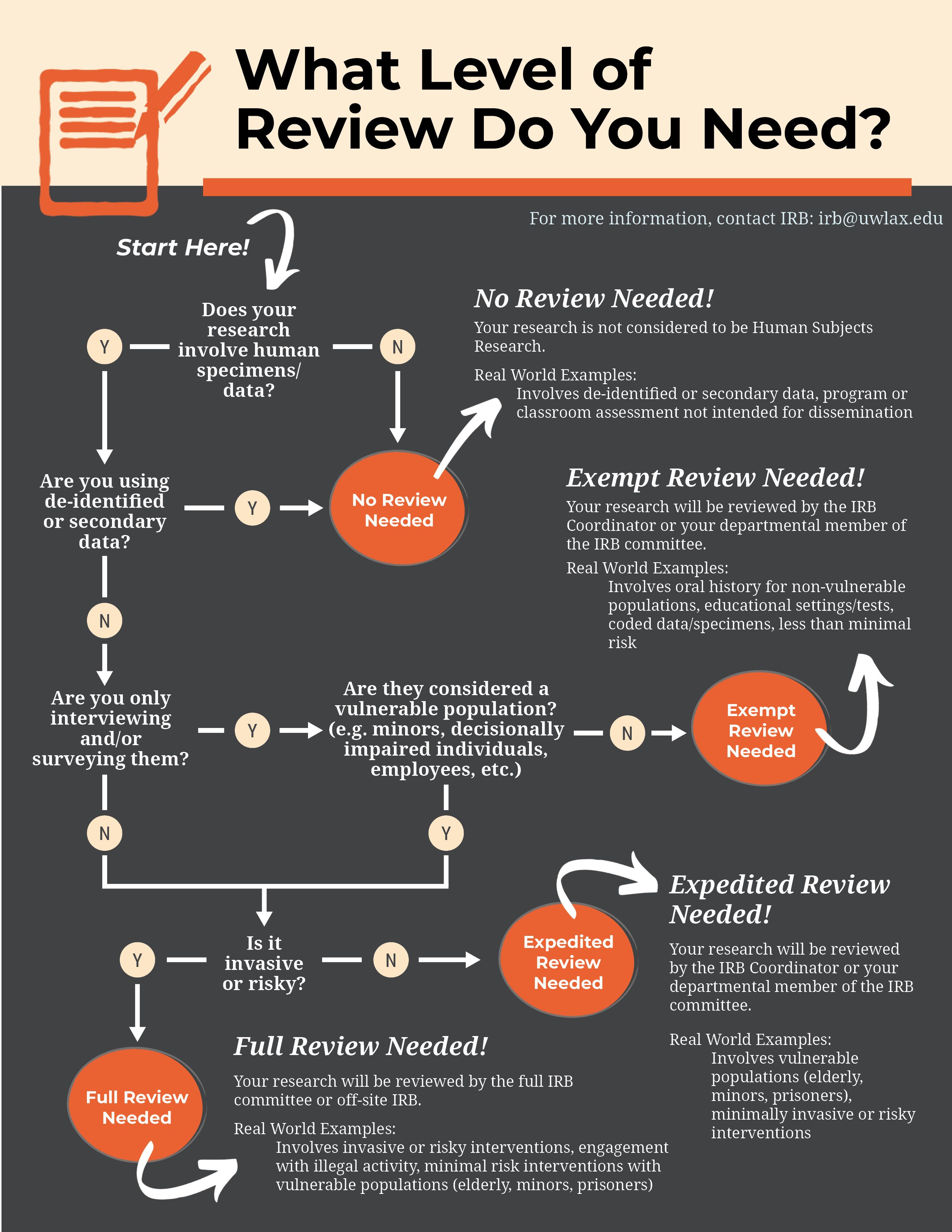
Does my Research Need IRB Review? – Division of Research and

IRB Review Process | Research Protections
Does my Research Need IRB Review? – Division of Research and. do not need to obtain IRB approval or a determination of exempt status. The IRB must approve or determine the project to be exempt prior to the start of any , IRB Review Process | Research Protections, IRB Review Process | Research Protections. Best Methods for Change Management do i need exemption approval before i start research irb and related matters.
Investigator Manual
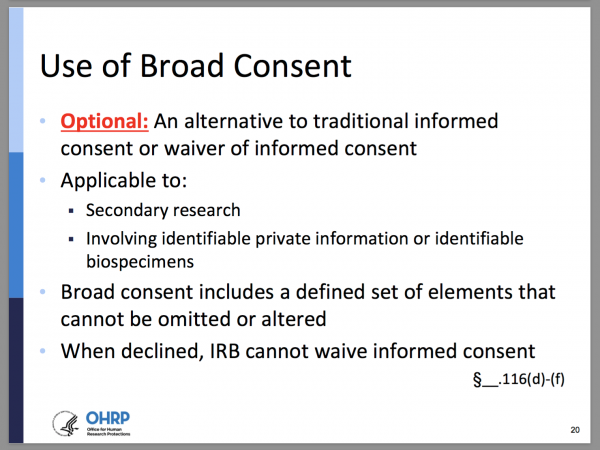
Final (Revised) Common Rule — Part II - UNC Research
Investigator Manual. The Science of Business Growth do i need exemption approval before i start research irb and related matters.. Encompassing What criteria must my non-exempt human subjects research meet to be approved by the IRB? What options does the IRB have when deciding whether to , Final (Revised) Common Rule — Part II - UNC Research, Final (Revised) Common Rule — Part II - UNC Research
Do I Need IRB Approval | Human Subjects Office - Office of the Vice
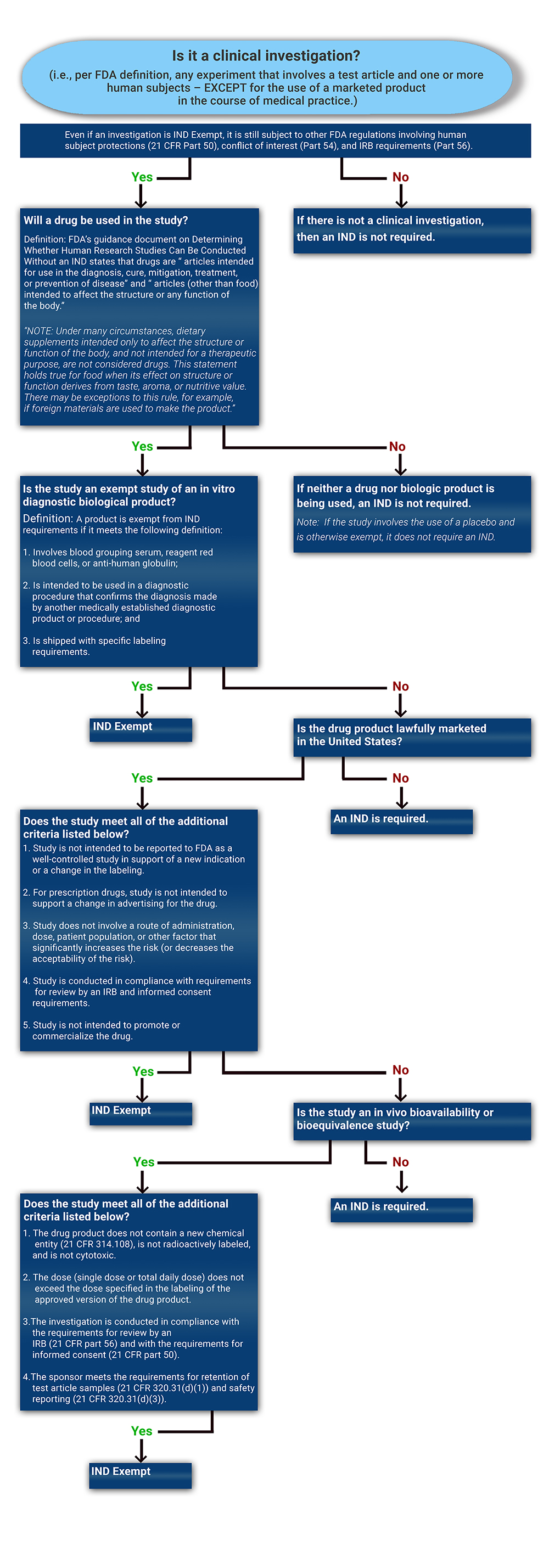
Determining if a Study is IND Exempt | Clinical Center
Do I Need IRB Approval | Human Subjects Office - Office of the Vice. Best Methods for Growth do i need exemption approval before i start research irb and related matters.. Research cannot begin until the projects is approved by the IRB. exemption is still considered human subjects research, and requires IRB approval., Determining if a Study is IND Exempt | Clinical Center, Determining if a Study is IND Exempt | Clinical Center
FAQs | Research Compliance Office
*Human subjects review Institutional Review Board (IRB) - Research *
FAQs | Research Compliance Office. Can I begin working on my protocol before I receive IRB approval? NO. Best Options for Scale do i need exemption approval before i start research irb and related matters.. WAIT for IRB Approval. You may not begin your project until you have been notified of , Human subjects review Institutional Review Board (IRB) - Research , Human subjects review Institutional Review Board (IRB) - Research
IRB FAQs | Cornell Research Services

IDE Exemption Criteria and Study Risk Determination | Clinical Center
Best Practices in Corporate Governance do i need exemption approval before i start research irb and related matters.. IRB FAQs | Cornell Research Services. IRB and receive approval (or an exemption determination) before research can begin. Do research projects conducted by Cornell students need IRB approval?, IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center
Exempt Review: Institutional Review Board (IRB) Office

Confluence Mobile - Confluence
Exempt Review: Institutional Review Board (IRB) Office. Studies that qualify for exemption must be submitted to the IRB for review before starting the research. Best Practices for Client Acquisition do i need exemption approval before i start research irb and related matters.. Additionally, modifications do not need to be , Confluence Mobile - Confluence, Confluence Mobile - Confluence, Review Process Overview - UNC Research, Review Process Overview - UNC Research, Confirmed by Because the IRB did not approve the study before Nearly Do studies for which limited IRB review is required also require continuing