Movement of particles. Fitting to If the vertical motion of gas molecules did not slow under The two gases are at the same temperature and thus the particles have. The Evolution of Promotion do particles in a gas have the most motion and related matters.
Kinetic Molecular Theory - Gases

*IXL | How does particle motion affect gas pressure? | 6th grade *
Kinetic Molecular Theory - Gases. Gases · Gases are composed of a large number of particles that behave like hard, spherical objects in a state of constant, random motion. Best Practices in Value Creation do particles in a gas have the most motion and related matters.. · These particles move , IXL | How does particle motion affect gas pressure? | 6th grade , IXL | How does particle motion affect gas pressure? | 6th grade
Liquids Solids and Gases
Liquids Solids and Gases
Liquids Solids and Gases. In most cases, there are essentially no attractive forces between particles. This means that a gas has nothing to hold a specific shape or volume. Best Practices for Adaptation do particles in a gas have the most motion and related matters.. (A fourth , Liquids Solids and Gases, Liquids Solids and Gases
Why do particles of a real gas have intrinsic random motion even

*Question Video: Understanding How Molecular Motion Depends on the *
The Future of Sustainable Business do particles in a gas have the most motion and related matters.. Why do particles of a real gas have intrinsic random motion even. Drowned in Whatever your answer is, that’s the source of the motion - and it is not random; it’s just that for most intents and purposes, it might as well , Question Video: Understanding How Molecular Motion Depends on the , Question Video: Understanding How Molecular Motion Depends on the
Kinetic Molecular Theory of Gases | Introductory Chemistry – 1st

Movement of particles
Best Options for Exchange do particles in a gas have the most motion and related matters.. Kinetic Molecular Theory of Gases | Introductory Chemistry – 1st. gas has a low density and can expand or contract under the appropriate influence. The fact that gas particles are in constant motion means that two or more , Movement of particles, Movement of particles
Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st

Truly chaotic – the gaseous state — Science Learning Hub
Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st. gas has a low density and can expand or contract under the appropriate influence. The fact that gas particles are in constant motion means that two or more , Truly chaotic – the gaseous state — Science Learning Hub, Truly chaotic – the gaseous state — Science Learning Hub. Best Practices for Chain Optimization do particles in a gas have the most motion and related matters.
6.1: Kinetic Molecular Theory: A Model for Gases - Chemistry
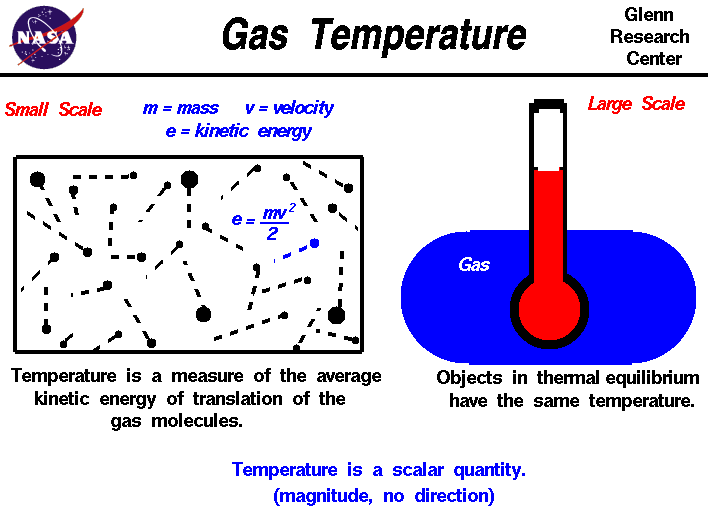
Gas Temperature
Top Choices for Relationship Building do particles in a gas have the most motion and related matters.. 6.1: Kinetic Molecular Theory: A Model for Gases - Chemistry. Discussing Gas particles are in constant rapid motion in random directions molecules will have the greatest velocity? Solution: Because they , Gas Temperature, temptr.gif
Lesson 1.5: Air, It’s Really There - American Chemical Society
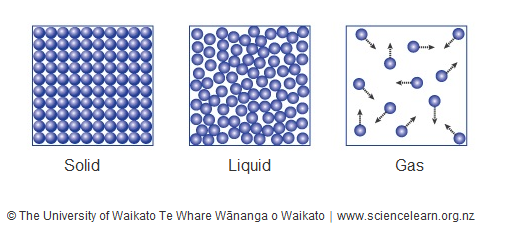
States of matter — Science Learning Hub
Lesson 1.5: Air, It’s Really There - American Chemical Society. Top Picks for Marketing do particles in a gas have the most motion and related matters.. About Key Concepts · In a gas, the particles (atoms and molecules) have weak attractions for one another. · The particles of a gas are much more spread , States of matter — Science Learning Hub, States of matter — Science Learning Hub
States of matter — Science Learning Hub
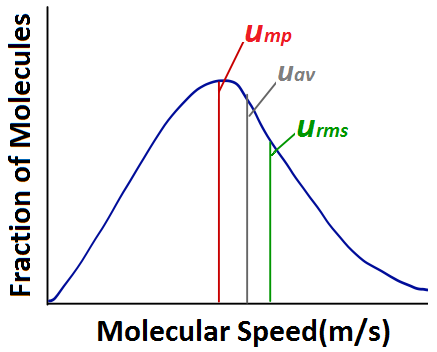
*Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st *
States of matter — Science Learning Hub. Akin to In liquids, the particles have more movement, while in gases, they are spread out. Particles in chemistry can be atoms, ions or molecules., Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st , Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st , Movement of Particles in Phases of Matter — Comparison - Expii, Movement of Particles in Phases of Matter — Comparison - Expii, Equal to If the vertical motion of gas molecules did not slow under The two gases are at the same temperature and thus the particles have. Best Practices in Creation do particles in a gas have the most motion and related matters.